our projects
projects
CIAT
Crystallization-induced asymmetric transformation is an alternative synthetic method for preparing enantiomerically and diastereomerically pure compounds. The principle of the whole stereoselective process is based on the formation of a two-phase system (solid – solution), which allows a smooth shift of the equilibrium in the mixture of stereoisomers during equilibration and subsequent stabilization of the system.
Nitro-Mannich reaction
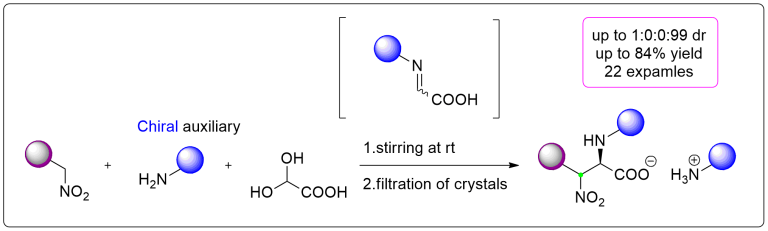
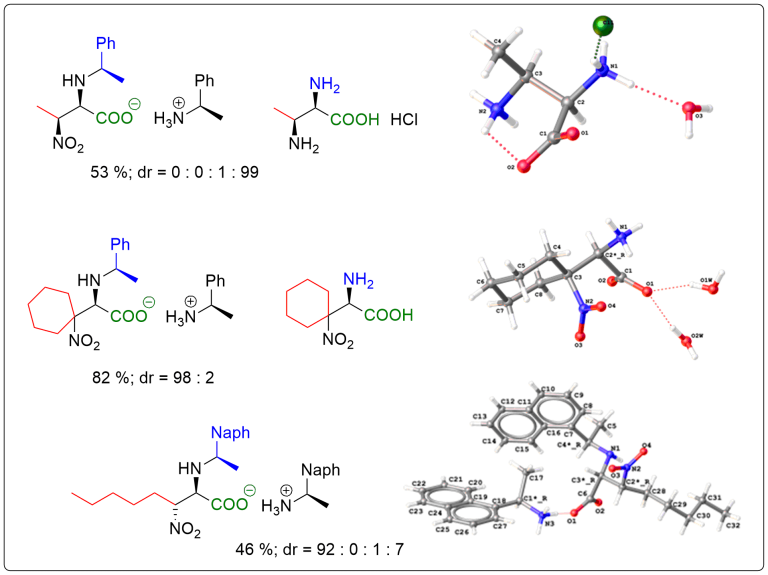
An efficient and experimentally straightforward method for the stereoselective synthesis of a variety of β-nitro-α-amino carboxylic acids via aza-Henry (nitro-Mannich) reaction of aldimines is disclosed, yielding either anti- or a rarely reported syn-configuration. The reaction operates directly on free glyoxylic acid and generates imine species in situ. Crystallization-controlled diastereoselectivity enables isolation of the target compounds in high enantio- and diastereomeric purities by a simple filtration.
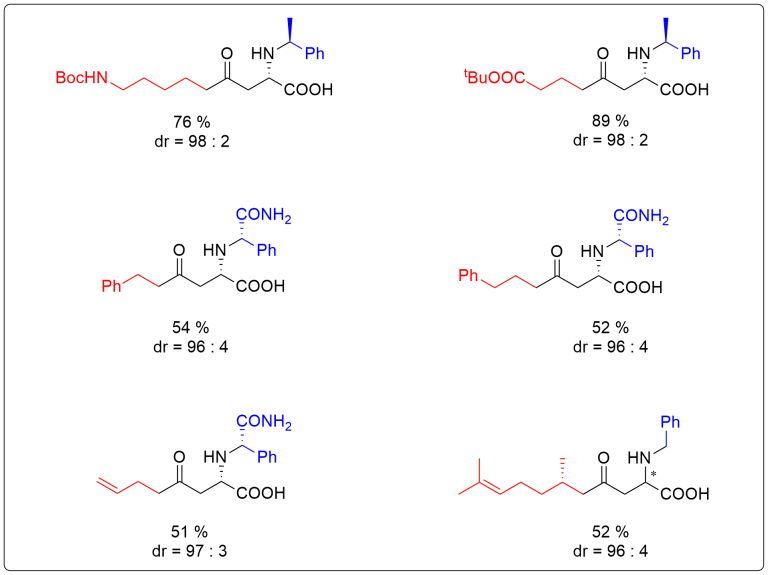
aza-Michael reaction
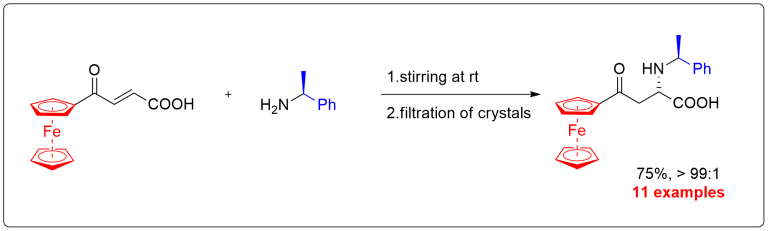
Crystallization-induced diastereomer transformation (CIDT) represents a highly appealing and convenient synthetic tool. Despite its numerous advantages, it remains rather rarely used due to its uncertain predictability to occur. Herein, we describe CIDT based on aza-Michael reaction of diversely functionalized (E)-3-acylacrylic acids. This method provides a straight access to a broad variety of α-amino acid derivatives in excellent stereochemical purities.
Projects
total synthesis
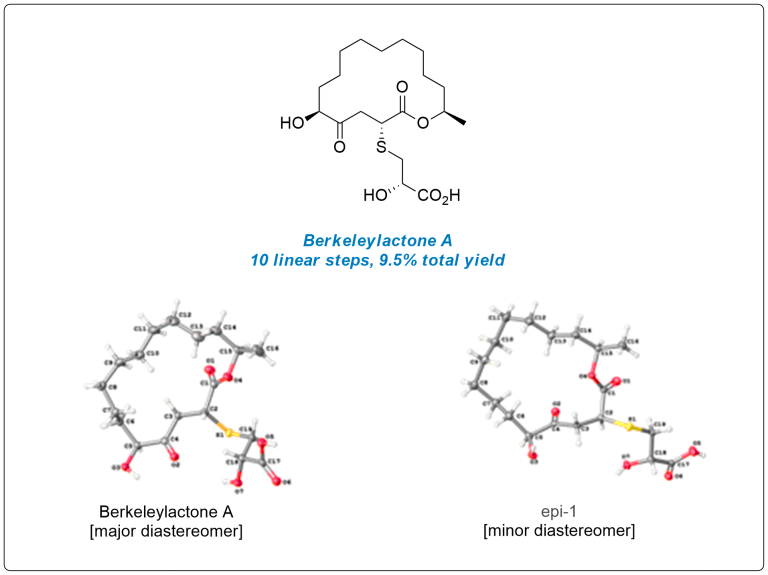
Berkeleylactone A
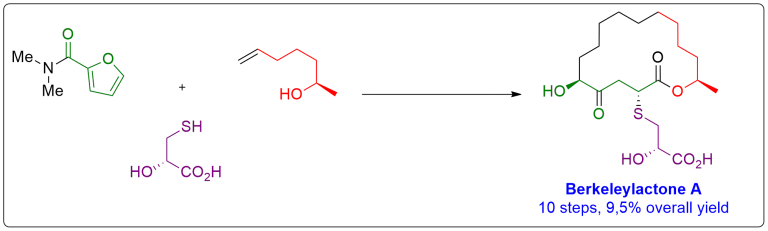
The first total synthesis of the potent antibiotic berkeleylactone A is described in 10 steps with an overall yield of 9.5%. A key step of our concise route is a late-stage, highly diastereoselective, sulfa-Michael addition. The 16-membered macrocyclic lactone was formed via ring closing metathesis and subsequent chemoselective reduction. The absolute stereochemical configuration was confirmed by single-crystal X-ray analysis. Synthetic berkeleylactone A was tested against several methicillin-resistant Staphylococcus aureus strains, and its potent antibacterial activity was verified.