REFERENCES
References
Prof. Andrew G. Myers, Department of Chemistry and Chemical Biology,
Harvard University, 12 Oxford St Cambridge, MA, United States;
myers@chemistry.harvard.edu; tel: +01 617-495-5718
Prof. Dr Darren J. Dixon, Department of Chemistry, Chemistry Research
Laboratory, University of Oxford, 12 Mansfield Road, Oxford OX1 3TA,
United Kingdom;
darren.dixon@chem.ox.ac.uk, tel: +44 (0) 1865275648, Fax: +44 (0)
1865285002
Assoc. Prof. Dr Dušan Berkeš, Slovak University of Technology,
Radlinského 9, Bratislava, Slovakia; dusan.berkes@stuba.sk tel: +421
(33) 7361769, +421 (2) 59325147, fax:+421 33 736 1951
Prof. Dr Tibor Gracza, Slovak University of Technology, Radlinského 9,
Bratislava, Slovakia;
tibor.gracza@stuba.sk tel: +421 (2) 52495410, fax: +421 (2) 52968560
Dr Kai Rossen, Director Synthesis Support, Sanofi-Aventis, Chemical
Sciences, Industriepark Hoechst, Building G 838, 65926 Frankfurt,
Germany;
kai.rossen@sanofi-aventis.com, tel: + 49 (0) 69 305 30203


BIOLOGICAL ACTIVE COMPOUNDS
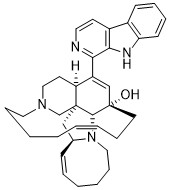
Manzamine A
anti-HIV-1, anti-inflammatory, anti-tumour activities, anti-bacterial,
anti-fungal, potent anti-malarial activities
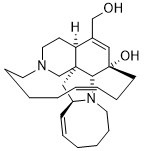
Ircinol A
exhibits antimalarial activity against both chloroquine-sensitive
and resistant strain of P. falciparum
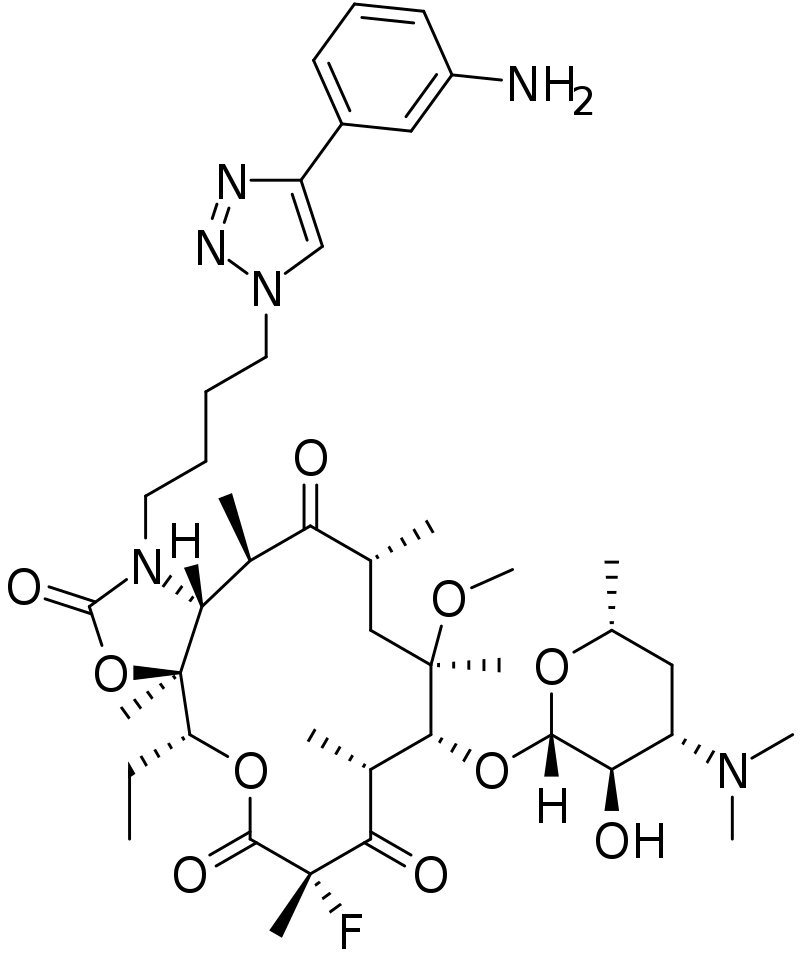
Solithromycin
- ketolide antibiotic undergoing clinical development for treatment of community-acquired pneumonia and other infections
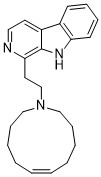
Manzamine C
anti-malarial activities
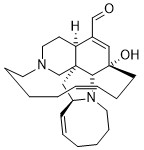
Ircinal A
documented antimalarial activities
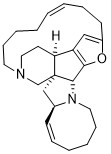
Nakadomarin A
cytotoxic – L1210, antimicrobial activity against a Gram-positive bacterium – Corynebacterium xerosis